Oxides: The Building Blocks of Glazes
Atoms may be the building blocks of the universe, but for pottery purposes the basic unit is an oxide, a molecule consisting of an element plus oxygen. Oxygen is so pervasive and reactive that it's easiest to think of elements in terms of its oxygenated molecule.
So in glaze parlance you would talk about Sodium (Na) as Soda (Na2O). Silicon (Si) as Silica (SiO2), Potassium (K) as Potash (K2O), Aluminum (Al) as Alumina (Al2O3), Magnesium (Mg) as Magnesia (MgO) and so on. Except for some outliers like Flourine, an element in pottery is usually attached to oxygen, and together they are an oxide. These oxides are the main characters in glaze chemistry. They are the building blocks of glazes.
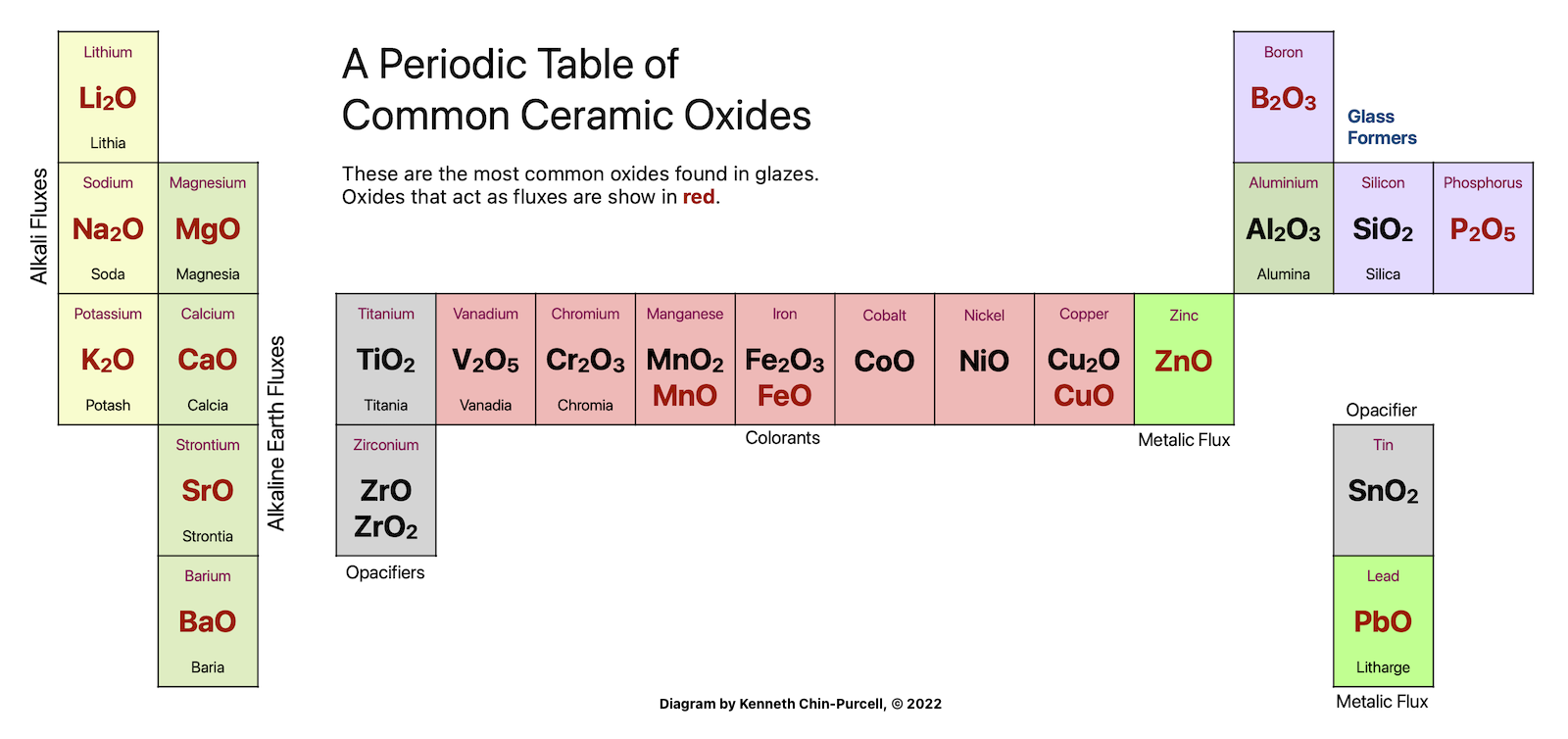
Elements like to pair up with oxygen in different ratios. Some prefer two atoms to one oxygen, like Soda (Na2O) and Potash (K2O). Others pair one to one, like Calcia (CaO) and Magnesia (MgO). A ratio of two to three is how Alumina (Al2O3), Boron (B2O3) and Iron (Fe2O3) like to pair. Silica and Titania pair up at one to two (SiO2 and TiO2).
Often oxides can be grouped according to how they pair with oxygen. So for example the 2:1 oxides, like Soda (Na2O) and Potash (K2O), have similarities. They are strong fluxes that also expand and contract a lot.
Glazes are mostly composed of silica and alumina, but silica and alumina by themselves are fairly inert. To get those oxides to melt at kiln temperatures we introduce fluxes, like soda, potash and calcia. These fluxes cause the alumina and silica to melt at lower temperatures. The ratio of fluxes to the rest of the glaze is a key to understanding how a glaze melts.
There is also a herd of additive oxides, like iron oxide (rust), copper oxide, and titania, that add color and texture.
The common oxides we see in glazes are also incidentally what the world is made out of. Your average mountain is mostly silica and alumina, combined with other oxides into various rocky minerals. A very common type of mineral is feldspar, where one of the flux oxides is bound up with alumina and silica. Your clay supplier probably carries a variety of soda and potash feldspars, along with more exotic feldspars like spodumene, a lithium feldspar.
A feldspar over eons of weathering might decompose, with the fluxes being washed away to the sea. You might wind up with just some alumina, silica and chemical water bound together in tiny platelets. That's clay.
So you can see why a feldspar might be a good starting point for getting the basic oxide ingredients you need for a glaze. Add some more silica and clay (for alumina) as needed, some flux material to get it all to melt, maybe an additive for color, and voila, you have a glaze!
Oxide Roles
Most glazes are over half silica. Silica is the backbone of any glaze.
Alumina stiffens up a glaze. Transparent glazes often have a silica to alumina ratio of about 9:1. Too much silica and the glaze might not melt, or if it does it might be very runny. Too little silica and again the glaze might not melt, or when it does there will be an excess of alumina giving a dry matte finish.
Soda and Potash act similarly and are strong fluxes. They also expand a lot when heated, causing crazing when the glaze cools. Lithia is also in this group of Alkaline Fluxes, the fluxes that have a 2:1 element to oxygen ratio.
Calcia, Magnesia, Baria and Strontia are the Alkaline Earth Fluxes. These fluxes have a 1:1 oxygen ratio. They may not be quite as strong as the Alkaline Fluxes but they also expand less. An excess of any of these fluxes will create a matte glaze.
Lead and Zinc oxides are Metallic Fluxes.
Boric Oxide (B2O3) is unique and very useful. It may not formally be a flux but it acts in concert with other oxides to lower the melting temperature and also lower expansion. It combines with oxygen at the same ratio as silica, 2:3.
Other oxides like Titania, Iron Oxide, and various metals (Copper, Cobalt) are used to change the color and surface of a glaze.
There is a special oxide in Mixup that isn't an oxide at all: LOI. LOI stands for Lost On Ignition, and it's a stand in for the stuff in a material that burns up in the kiln. It has an oxide weight of zero. Carbon for example won't survive a kiln firing, so calcium carbonate (whiting) leaves behind calcia in the glaze, with the carbonate bubbling off into the kiln atmosphere. You can add LOI to a material analysis to make it tally to 100% but it won't show up in the oxide analysis.
